ICDH
- Isocitrate dehydrogenase from Burkholderia pseudomallei:
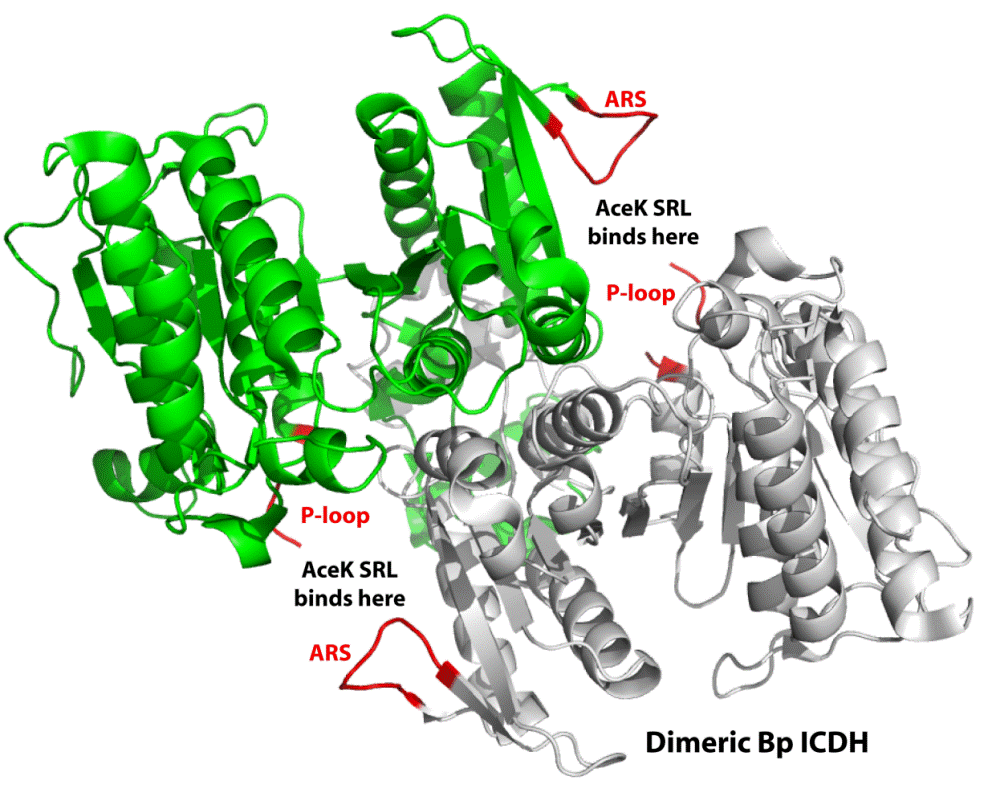
In collaboration with researchers from Queen’s University, scientists from the Seattle Structural Genomics Center for Infectious Disease (SSGICD) have reported the crystal structure of a key protein from the citric acid cycle or Kreb’s cycle, which is an essential enzyme for all life. This enzyme, isocitrate dehydrogenase (ICDH), converts isocitrate to a-ketoglutarate and carbon dioxide in the third step of the citric acid cycle. The structure (BupsA.00092.a, PDB entry 3DMS) of ICDH from Burkholderia pseudomallei (Bp), a Gram-negative bacterium that causes acute and chronic melioidosis, exhibits structural elements different from human ICDH. These differences allow Bp ICDH to be modulated by the kinase AceK and may be exploited for drug development against the disease melioidosis. For example, knowledge of the differences between the pathogen and human enzymes will facilitate targeting the pathogenic enzyme while avoiding interactions with the human enzyme.
The structure and accompanying kinetic analyses were published on August 26, 2011 in the journal Biochemistry as an accelerated publication by Susan P. Yates et al. titled “Structural basis of substrate specificity of bifunctional isocitrate dehydrogenase kinase/phosphatase.”
Reference
“Structural basis of substrate specificity of bifunctional isocitrate dehydrogenase kinase/phosphatase.” Susan P. Yates, Thomas E. Edwards, Cassie M. Bryan, Adam J. Stein, Wesley C. Van Voorhis, Peter J. Myler, Lance J. Stewart, Jimin Zheng, and Zongchao Jia. Biochemistry 2011, published online as an Accelerated Publication August 26, 2011.
PMID: 21870819.