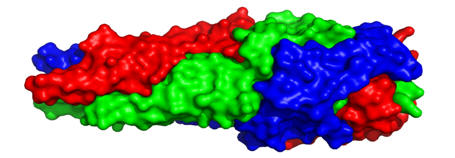
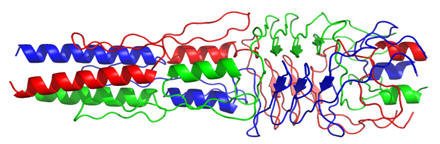
xadA
- xadA Trimeric Autotransporter Adhesin Head:
Adhesion of the pathogen to the host is a crucial first step in infection. The structure presented here is for the head domain of protein xadA Trimeric Autotransporter Adhesin (TAA) from Burkholderia pseudomallei. This pathogen is not only a potential bioterror agent but also causes melioidosis, which results in skin infections, chest, bone and joint pain, cough, lung nodules and enables contraction of pneumonia. TAAs diverge greatly in sequence and domain structure across species, however they are most commonly comprised of a c-terminal membrane anchoring domain and a passenger domain. The passenger domain is believed to pass through the membrane domain on its way to the outside of the pathogen’s cell wall, where it contributes to the adhesion of the bacteria to the host cell surface. Within the passenger domain, the head portion of this protein contains three regions (FGG, HANS and HIM-2) which were not previously described structurally. The importance of this protein for pathogen virulence, combined with its close association with the pathogen cell membrane (where it is more accessible), make it a potential target for drug development. Additionally, a structural understanding of the FGG, HANS and HIM-2 regions of the protein will allow for improved computational modeling, which is defined as structure elucidation from sequence alone. For more information, please see the Protein Data Bank entry 3la9 and 3laa.